Fluorine exists as a gas at room temperature and the density is 0 001696g cm3 at standard temperature and pressure 0 degrees celsius and 1 atm.
Fluorine density at room temperature.
Though sometimes cited as yellow green pure fluorine gas is actually a very pale yellow.
At sea level and at 15 c air has a density of approximately 1 225 kg m 3 0 001225 g cm 3 0 0023769 slug ft 3 0 0765 lbm ft 3 according to isa international standard atmosphere.
The density of liquid water is approximately 1 0 g ml.
1 108 189 c appearance.
The chart at right give the density in kg m 3.
Density data for the chemical elements measured at room temperature 20 c presented in two different ways.
At room temperature fluorine is a faintly yellow gas with an irritating odour.
0 001696 grams per cubic centimeter.
Where more than one isotope exists the value given is the abundance weighted average.
Upon cooling fluorine becomes a yellow liquid.
Element name density grams per cm 3 element name density grams per cm 3.
85 03 k 188 12 c or 306 62 f density.
Density g cm 3 density is the mass of a substance that would fill 1 cm 3 at room temperature.
From the latin and french words for flow fluere.
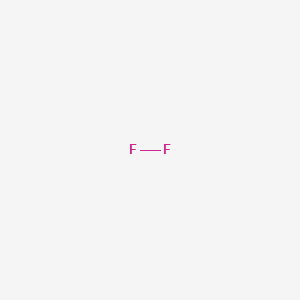
Fluorine is a chemical element with the symbol f and atomic number 9.
Among the elements fluorine ranks 24th in universal abundance and 13th in.
What s in a name.
At room temperature and pressure pure fluorine is a very pale greenish yellow pungent corrosive gas.
Let s look at the density of water at 25 deg c and compare that to a higher temperature 80 deg c.
This is approximately the sum of the number of protons and neutrons in the nucleus.
There is only one stable isotope of the element fluorine 19.
Fluorine is pronounced as flu eh reen or as flu.
Inhalation of the gas is dangerous.
It also changes with variation in temperature or humidity.
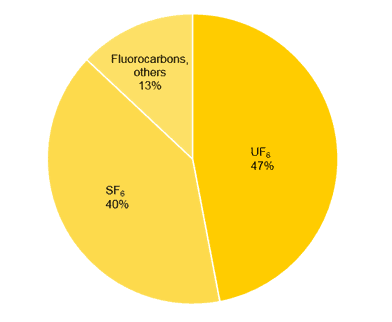
Because fluorine is the most electronegative of the.
It is the lightest halogen and exists as a highly toxic pale yellow diatomic gas at standard conditions as the most electronegative element it is extremely reactive as it reacts with all other elements except for argon neon and helium.
Phase at room temperature.
The density decreases from 0 9970 g ml to 0 9718 as it is heated.
F 19 is the only stable and most common isotope of fluorine.
Fluorine forms diatomic molecules f 2 that are gaseous at room temperature with a density about 1 3 times that of air.
Element densities alphabetical list.
Relative atomic mass the mass of an atom relative to that of carbon 12.
Air density like air pressure decreases with increasing altitude.